Polarity in Covalent Bond
Polarity in Covalent Bond: Overview
This topic discusses the classification of the covalent bond based on the electronegativity of the atom. It explains the concept of the polarity of a bond, its cause and dipole moment. It highlights the dipole moment of molecular structures.
Important Questions on Polarity in Covalent Bond
Which of the following compounds, hydrocarbons, has the lowest dipole moment?
Which of the following molecules is polar
Give the formula to calculate percentage of covalent character from ionic character?
If is the magnitude of charge and is the distance between the centres of positive and negative charges then dipole moment is given by
CCl4 has zero dipole moment
The dipole moment of the given molecules are such that :
A covalent molecule, , is found to have a dipole moment of C m and a bond length of pm. The percent ionic character of the bond will be
A neutral molecule has zero dipole moment. The element is most likely?
Which of the following hydrocarbons has the lowest dipole moment?
Which one of the following has the highest dipole moment?
Assertion. Boiling points of cis-isomers are higher than trans-isomers.
Reason. Dipole moments of cis-isomers are higher than trans-isomers.
Molecule which has non-zero dipole moment and planar is:
What is the magnitude of charge on central atom if for the given molecule which is a linear molecule, the dipole moment of bond is 0.38 D and bond distance is
If there is a molecule which has equal dipole moment as that of toluene. Then, is?
The number of orbital dipoles is equal to the number of the bond moments in which of the following option.
Incorrect statement for molecule is:
About the dipole moment of given molecules, which option is correct?
Find out the correct order of dipole moments for the molecules given below:
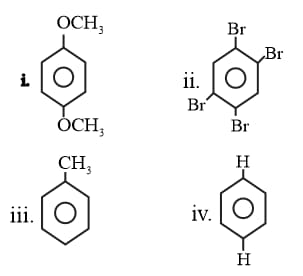
Find the minimum polarity compound among the following.
(i)
(ii)
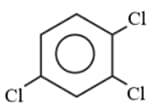
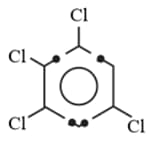
Observe the above molecules and find out the ratio of dipole moment of I and II if dipole moment of bond is .
